Exploring the Quantum World with SIESTA
What is Quantum Physics about?
-

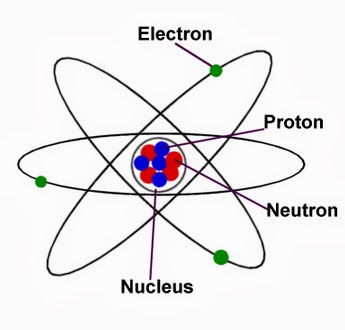
Atom Structure: Nucleus (protons+neutrons) and Electrons (credits: Wikipedia)
In a “tiny” nutshell, the Quantum World is made of atoms and molecules (i.e. atoms bonded together). Atoms can be seen as little beads, however they have an internal structure: a nucleus made of protons and neutrons, and an electron cloud in the outer parts (see representation on the right). The nucleus constituents are thousands of times heavier than the electrons and are located in the center. The nucleus has a positive charge carried by the protons (neutrons are neutral as the name indicates it). Since the atom is neutral, these positive charges are compensated by the negative ones carried by the electrons. There is a famous equation which describes how this tiny “things” behave: the Schrödinger Equation. It links the dynamics and the energy of the system in a very powerful manner!
Nature Building Blocks
These are the building blocks of which matter in Nature is made (our body, the water you drink, supercomputers, etc.). Once we know the rules to assemble atoms, we can play this game and try to bind hundreds and even thousands of atoms together. It turns out that, for bulk materials, we can recognize patterns of several atoms repeated over the space in different directions (lattice vectors). At this stage, we are able to study how materials are formed and which properties they have (conductivity, resistivity, etc.). Crystals are solids made of these repeated unit cells and there is an extended collection of them, which are classified by their symmetry properties. Salt (NaCl) is a good crystal example we use every day. Below you can see how Sodium (Na) atoms are associated with Chlorine (Cl) ones in order to build this basic cell.
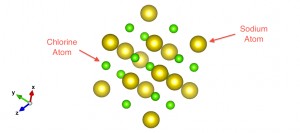
Salt (NaCl) Unit Cell: Sodium atoms (green) and Chlorine atoms (yellow)
Quick Insight into Shcrödinger Equation (optional)
The scope of our project is to model such structures and study how the electrons distribute around different molecules and crystals. The tricky thing is that, for theoretical reasons, we are not able to exactly solve the Schrödinger equation for systems bigger than Hydrogen… and Hydrogen is just made of one proton and one electron! The generic time-dependent Schrödinger Equation can be written in the following manner:
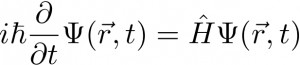
where Psi (the Greek letter) represents the state of the system as a function of space (vector r) and time t. We do not need to care about the constants on the left side, just note that we are looking at the variation over time by taking the derivate. Finally, H stands for the Hamiltonian (which contains the dynamics) of the system we are considering. H can be split into a kinetic part (velocity dependent) and a potential part (usually called V), these are strongly dependent on the number of particles (protons, neutrons and electrons) and can be very complex. For instance, if we write the Schrödinger Equation for the Hydrogen, without magnetic field and for low velocities (non-relativistic case), we only need to consider the electron-proton and proton-electron interactions. This leads to the following equation:
![]()
![]()
We use here the 2-body reduced-mass of the system and the Coulomb (electric) interaction V only, where e is the electrical charge of the electron. E stands for the Energy of the {proton-electron} system here. I wouldn’t like to go into detail since further mathematical steps would only scare people. You only need to know that, by applying variable separation and using some trendy functions called Spherical Harmonics, this equation can be exactly solved. This means that we obtain the energy spectrum of Hydrogen (as Schrödinger itself did in 1926) which can be measured experimentally.
You may ask yourself how can we treat bigger atoms and molecules then… This issue constraints us to do some approximations if we want to move further. Nevertheless, the accuracy of the calculations compared to experiments remains spectacular! Quantum Physics also teach us that only outer electrons are responsible for the forces which bind molecules together, the so-called valence atoms. Let’s see now how we can play with molecules and crystals!
SIESTA: a very powerful tool!
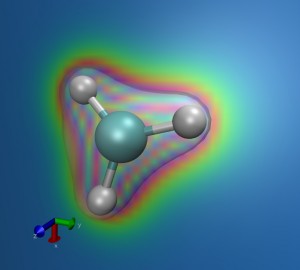
CH3 rendering: the Carbon atom is in the middle and the 3 Hidrogen atoms are bounded to it. The colored slice represents the electron density on this plane.
SIESTA (no, it has nothing to do with “nap” in Spanish) is a parallel code which enables us to compute the distribution of electrons around complex systems using MPI. Instead of calculating the position of every electron, it only considers the outer ones which are involved in the chemical bindings and leave the atoms almost fixed during the simulations. Moreover, electrons are not considered as tiny beads, but as diffuse entities over the space. That means that we only know the probability density of electrons around the molecule, no matter where the electrons really are (funny Quantum Mechanics!). This sophisticated technique is called Density Functional Theory (DFT). You can see on the left a simple example of a CH3 molecule and the electronic density obtained when we take a slice of the system. The darker colors mean that there is a higher probability to find the electrons in these regions. You can also see the shape of this distribution as a transparent surface on the top.
Future Work
Future works will be focused on more complex systems and study its electronic distribution in order to create nice visualizations. I’ll concentrate on Forsterite (Mg2SiO4) and Enstatite (Mg2Si2O6). I’ll tell you in a future post why! In addition, we would like to see the scalability of this code by running the same simulation on different machines with different number of processors. Tip of the day: Scalasca it a very powerful tool for this profiling task, if you would like to do something similar you should check it out.
I hope this “brief” insight into Quantum World was clear enough. If you have some questions, just leave a comment! I would be glad to answer.

Sorry, but how do you compute the eigen-functions within a finite potential well?
:-).
Cool!