And yet they moves!

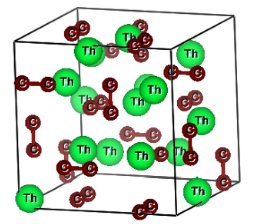
Thorium sesquicarbide (Th2C3) is a potential candidate as a nuclear fuel for generation IV advanced reactors. The microscopic structure of this compound is shown in the figure. Physicists call that cube unit cell of the material, i.e. the smallest portion of a crystal lattice that shows the pattern of the entire crystal: you can build your solid repeating this structure in all the three direction x,y,z.
Although a solid consists in billions and billions of atoms, the unit cell usually contains a few of them. For example, regarding Molybdenum (see previous blog) only 2 atoms are in the unit cell, for Palladium (see Mattia blog ) there are 4 of them. How can it be possible? How does a complicated problem reduce to a problem of few atoms? The answer is that there is a microscopic order, there are spatial symmetries that allow us to understand a solid starting from a small building block. However the unit cell of our compound is not so small, it contains 40 atoms (24 C and 16 Th). For this reason we need a supercomputer to perform all the calculations!
Electronic Properties
The first step to grasp the main features of this compound is to study how electrons behave, in particular how they make the bonds between the atoms and how the electronic charge is spatially distributed.
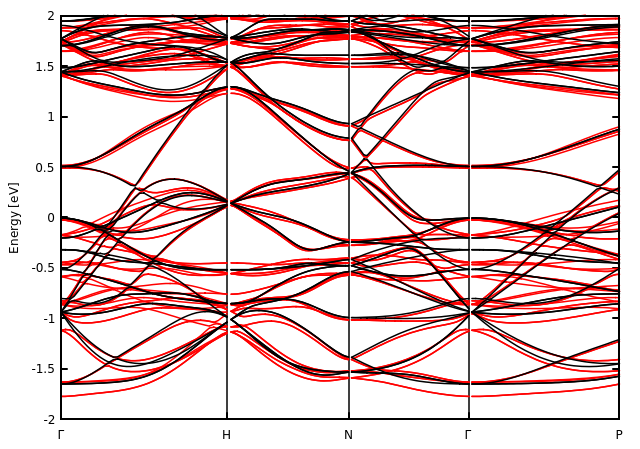
In the figure the so-called band structure of Th2C3 is reported. The vertical axis is the energy of the single electron with respect to a particular energy (Fermi energy). The horizontal axis is a quantum number defined from the periodicity of the crystal and related with the momenta of the electrons. The Fermi energy is set in such a way that it separates the filled states from the empty states. You have to imagine that in all the lines below 0 energy there are electrons while there are not above. So an electron near 0 requires an infinitesimal amount of energy to move in an empty state. This fact implies that the main properties of the material are determined on how many electrons are near the Fermi energy!
Lattice vibrations
Now that electronic problem is solved we can study how the ions move! The strategy is as follows:
- Find the equilibrium distance of the ions
- Make a little displacement of the ions to calculate the forces
- Use the periodicity to study only the problem in the unit cell
Below are shown some characteristic vibrations
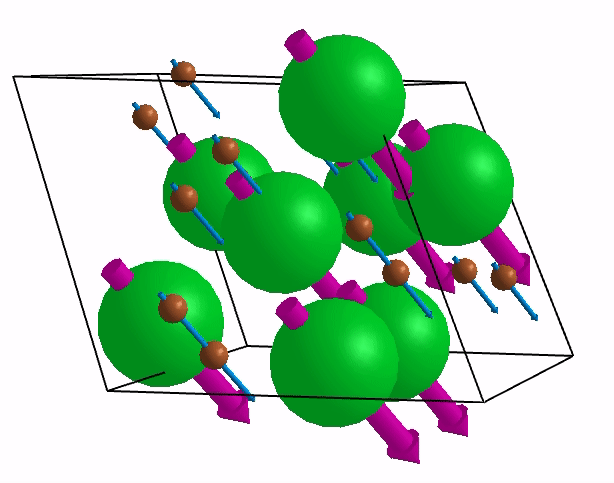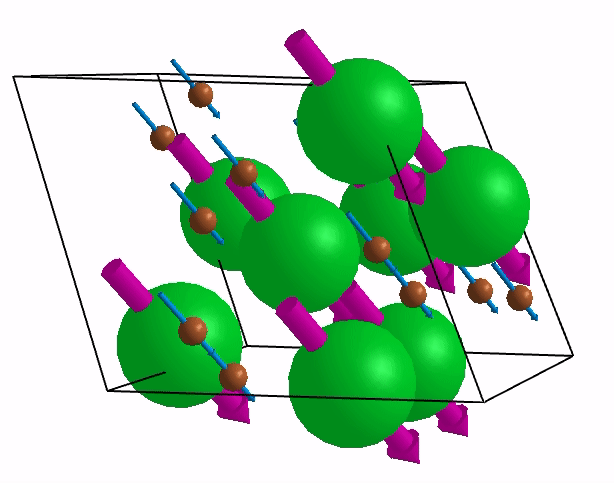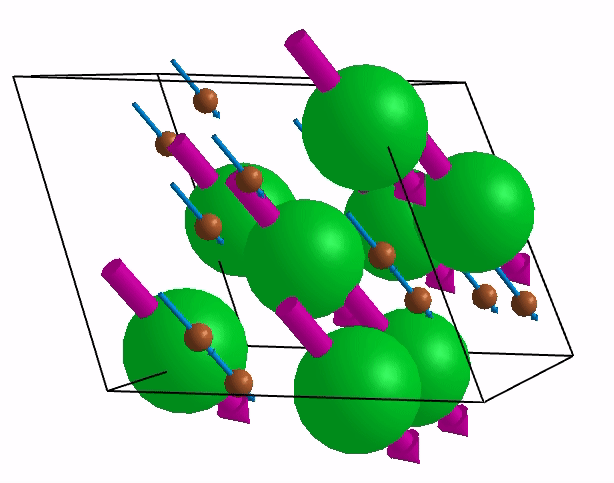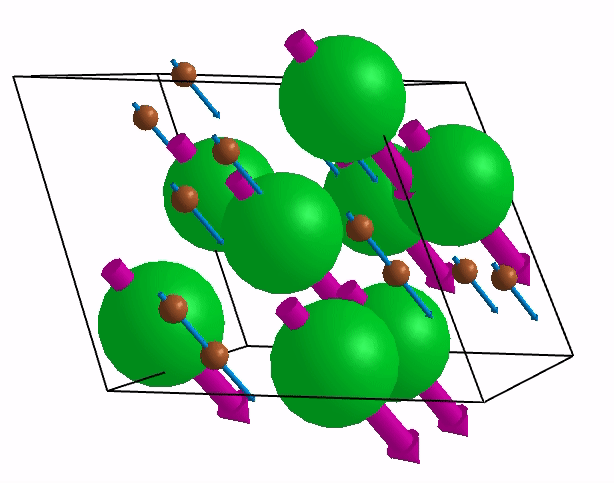
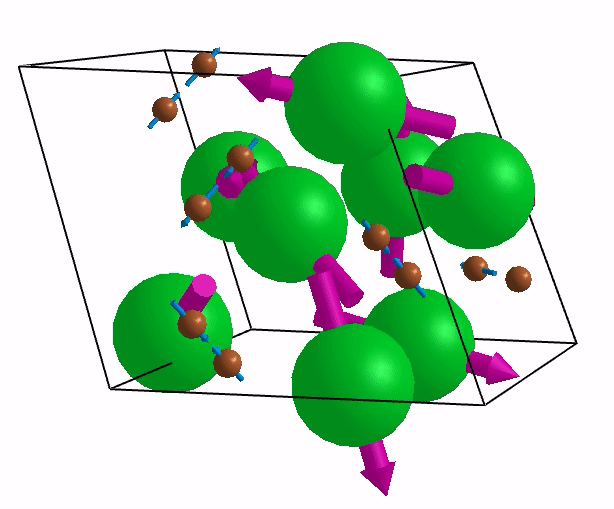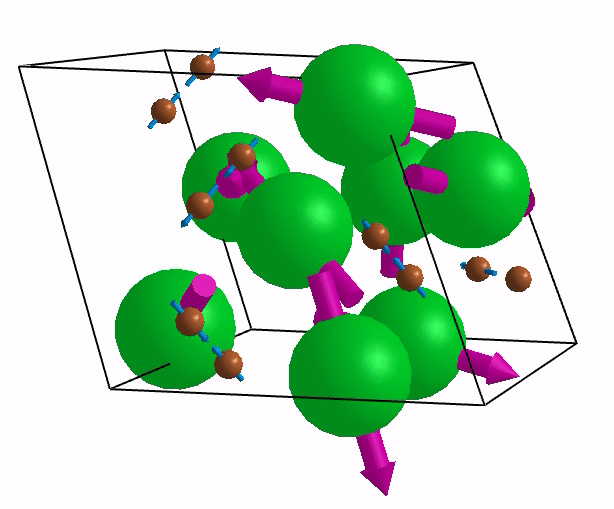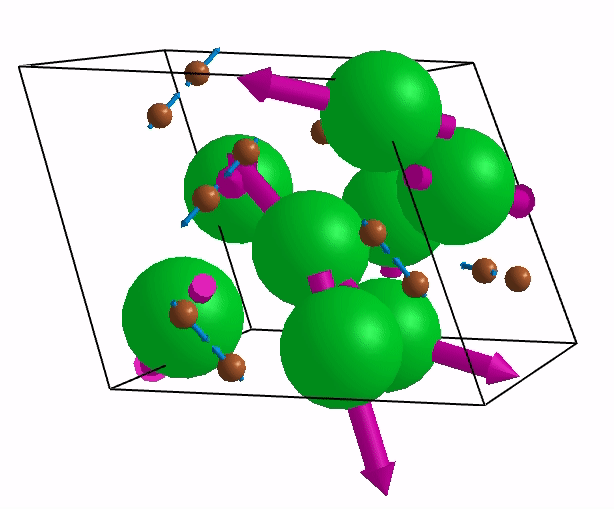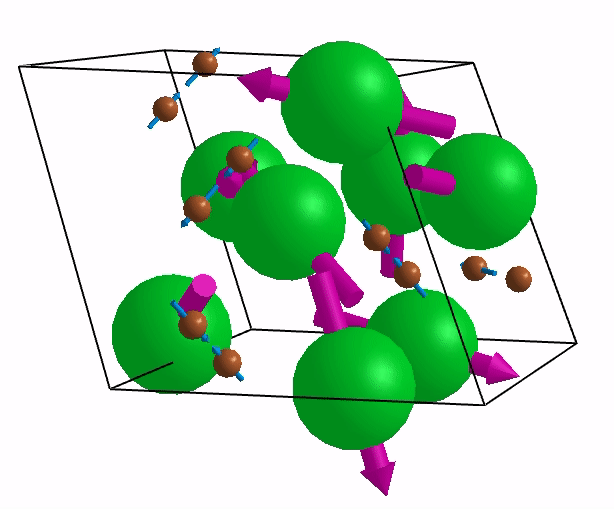
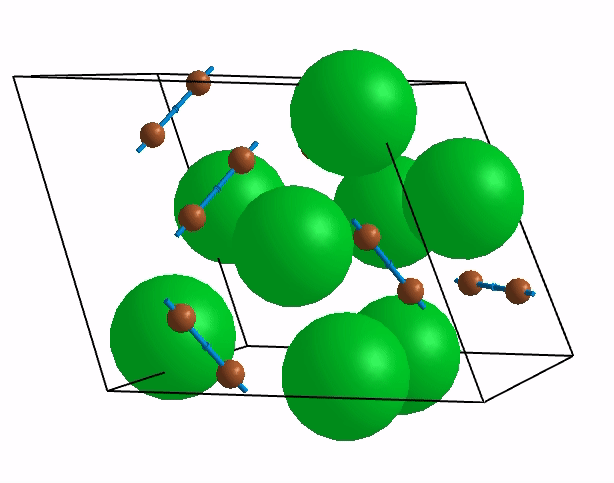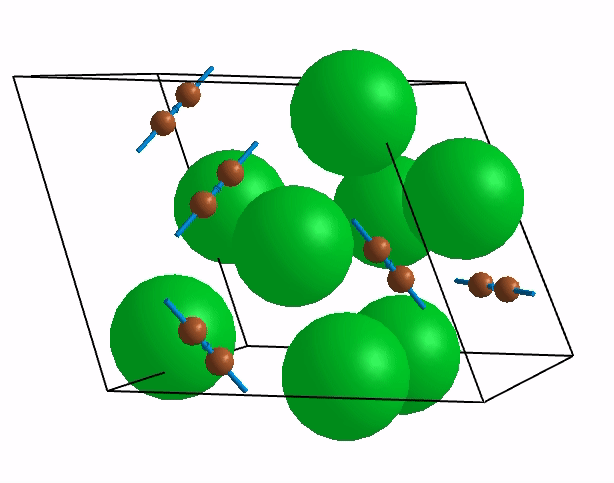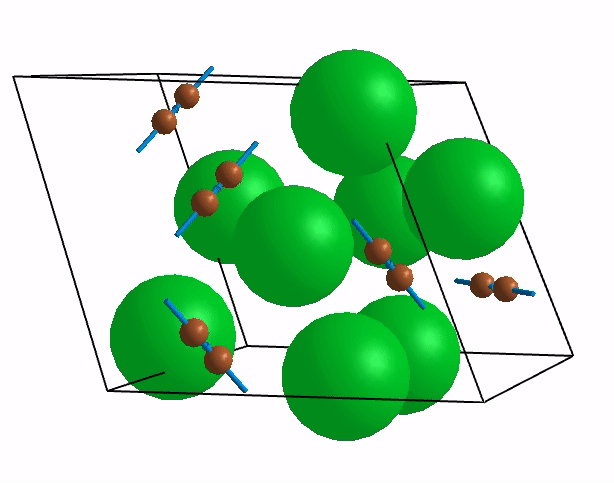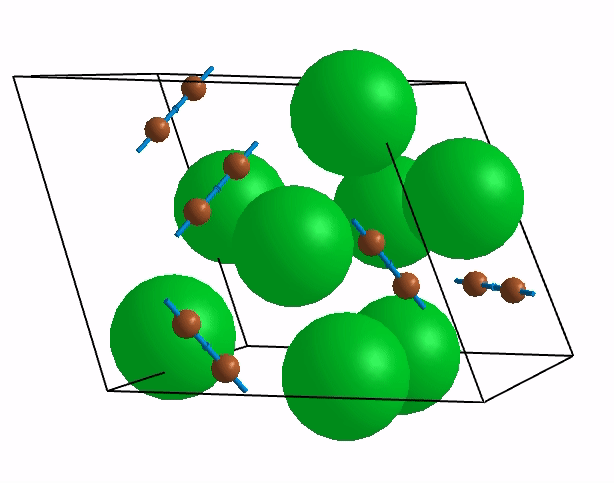
These vibrations combined with electronic motion will determine thermal properties of the potential novel nuclear fuel!
Acknowledgement
I’m really grateful to every person that made my partecipation for the Summer of HPC 2021 possible. It is a fantastic experience that I would recommend to anyone interested in computational science and its applications. I hope you found my blog pages interesting and helpful to understand how we can deal with materials from a physical point of view.
